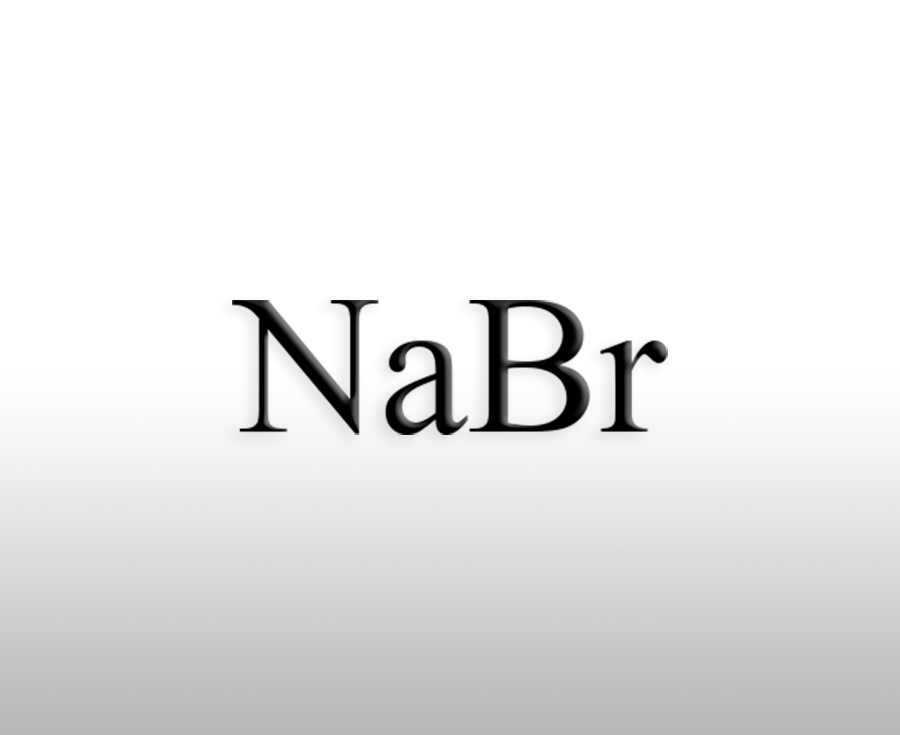News Center
Popular products
Sales Department Tel:
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
[email protected]
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
[email protected]
[email protected]
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
saxgroup.cn
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
[email protected]
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
[email protected]
[email protected]
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
saxgroup.cn
Procurement Hotline
0512-52836128
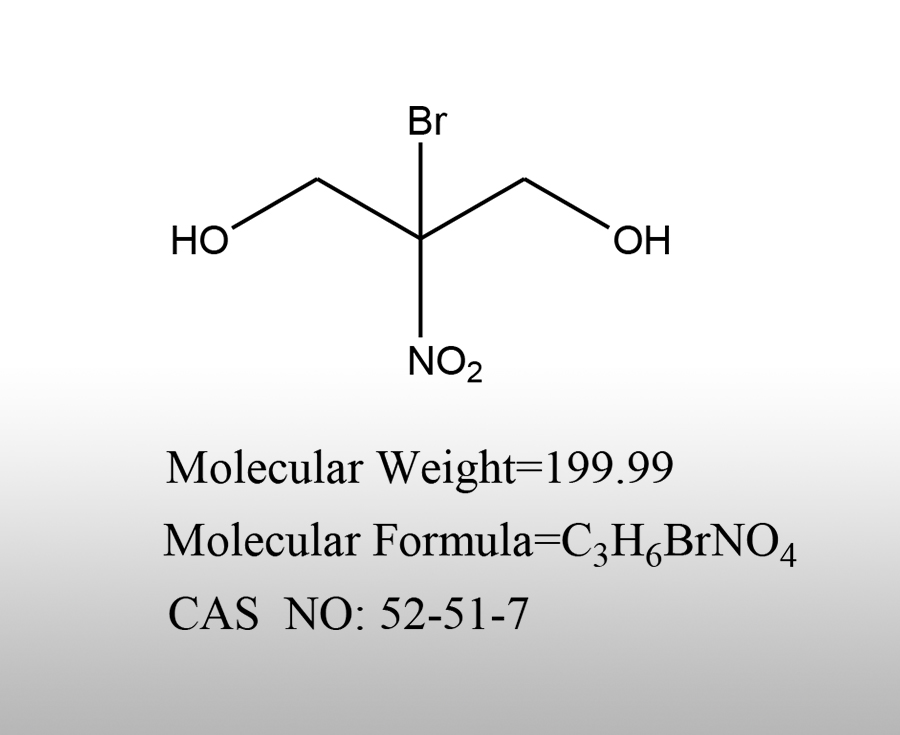
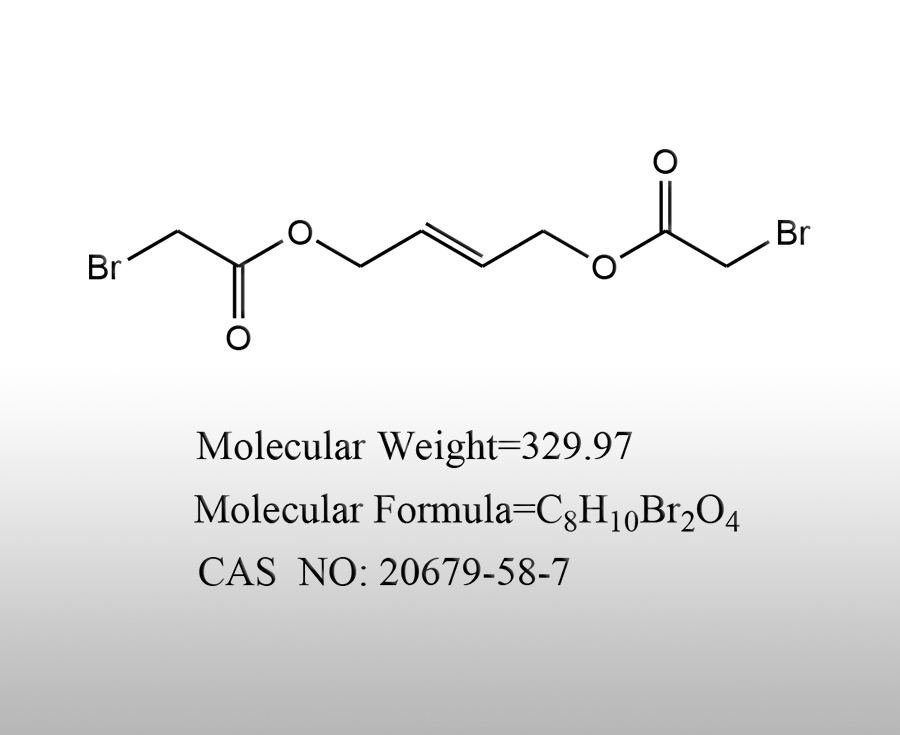
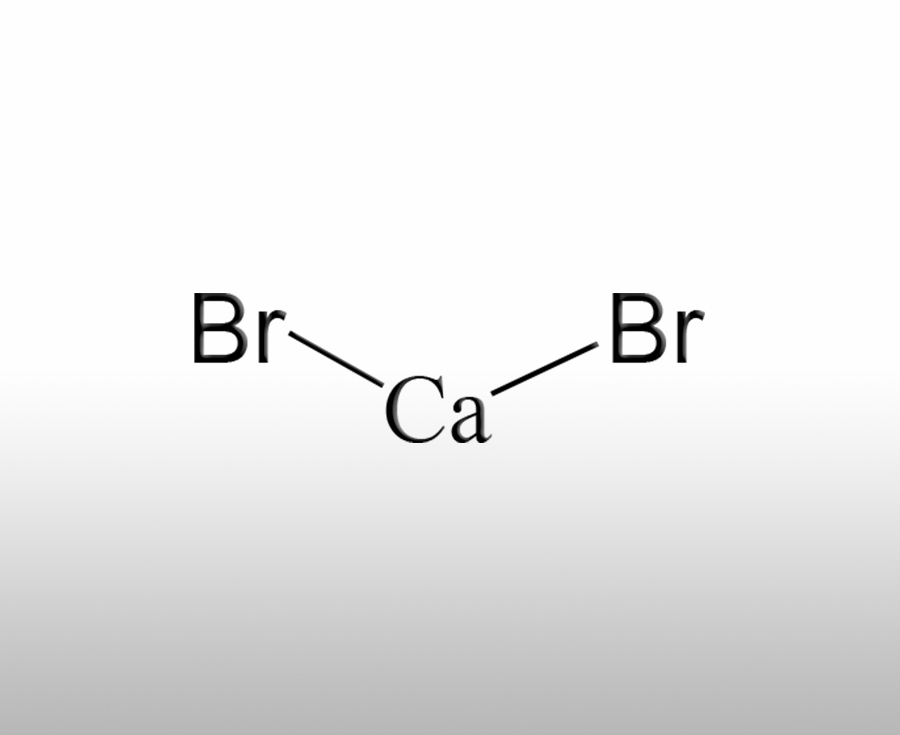
What is the use of calcium bromide
Calcium bromide is an important inorganic compound with a wide range of applications in various fields, including the following aspects:Sources:saxgroup.cn | PublishDate:2025.04.29
1. Petroleum drilling: Calcium bromide aqueous solution has high density and good solubility, and can be used as a weighting agent for drilling fluids during the petroleum drilling process. It can adjust the density of drilling fluid, balance formation pressure, prevent accidents such as blowouts and well collapses, and also play a role in suppressing the expansion and dispersion of clay minerals in the formation, stabilizing the wellbore.
2. Pharmaceutical field: Calcium bromide has a certain sedative effect and can be used as a sedative in medicine to treat diseases such as neurasthenia and epilepsy. It can suppress the excitability of the central nervous system, stabilize patients' emotions, and alleviate symptoms of tension and anxiety. In addition, it can also be used as a pharmaceutical intermediate for the synthesis of other drugs.
3. Photographic materials: In the photosensitive industry, calcium bromide is used to manufacture photosensitive materials such as photographic film and paper. It is one of the important components in photosensitive emulsions, which can interact with substances such as silver halide, affecting the sensitivity, contrast, and resolution of photosensitive materials, and helping to improve the imaging quality of photosensitive materials.
4. Chemical analysis: Calcium bromide can be used as an analytical reagent to determine the content of other substances. For example, in some chemical analysis methods, it can form precipitates or complexes with certain metal ions, and the content of metal ions can be determined by analyzing the precipitates or complexes. In addition, it can also be used to prepare other bromides.
5. Flame retardant: Calcium bromide can be used as a component of flame retardants and added to materials such as plastics, rubber, textiles, etc., which can improve their flame retardant properties. When the material encounters a fire source, the bromide ions produced by the decomposition of calcium bromide can capture the free radicals generated during the combustion process, thereby inhibiting the progress of the combustion reaction, slowing down the spread of fire, reducing the burning speed and fume emission of the material, and improving the safety of the material.
Prev:
None